Enrico Fermi
From Wikipedia, the free encyclopedia
"Fermi" redirects here. For other uses, see Fermi (disambiguation).
| Enrico Fermi | |
|---|---|
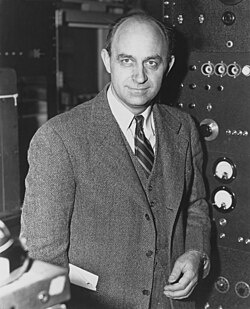
Enrico Fermi (1901–1954)
|
|
| Born | 29 September 1901 Rome, Italy |
| Died | 28 November 1954 (aged 53) Chicago, Illinois, United States |
| Citizenship | Italy (1901–54) United States (1944–54) |
| Fields | Physics |
| Institutions | Scuola Normale Superiore University of Göttingen Leiden University University of Florence Sapienza University of Rome Columbia University University of Chicago |
| Alma mater | Scuola Normale Superiore |
| Doctoral advisor | Luigi Puccianti[1] |
| Doctoral students | |
| Other notable students | |
| Known for | |
| Notable awards |
|
| Spouse | Laura Fermi |
| Signature | |
Fermi's first major contribution was to statistical mechanics. After Wolfgang Pauli announced his exclusion principle in 1925, Fermi followed with a paper in which he applied the principle to an ideal gas, employing a statistical formulation now known as Fermi–Dirac statistics. Today, particles that obey the exclusion principle are called "fermions". Later Pauli postulated the existence of an uncharged invisible particle emitted along with an electron during beta decay, to satisfy the law of conservation of energy. Fermi took up this idea, developing a model that incorporated the postulated particle, which he named the "neutrino". His theory, later referred to as Fermi's interaction and still later as weak interaction, described one of the four fundamental forces of nature. Through experiments inducing radioactivity with recently discovered neutrons, Fermi discovered that slow neutrons were more easily captured than fast ones, and developed the Fermi age equation to describe this. After bombarding thorium and uranium with slow neutrons, he concluded that he had created new elements; although he was awarded the Nobel Prize for this discovery, the new elements were subsequently revealed to be fission products.
Fermi left Italy in 1938 to escape new Italian Racial Laws that affected his Jewish wife Laura. He emigrated to the United States where he worked on the Manhattan Project during World War II. Fermi led the team that designed and built Chicago Pile-1, which went critical on 2 December 1942, demonstrating the first artificial self-sustaining nuclear chain reaction. He was on hand when the X-10 Graphite Reactor at Oak Ridge, Tennessee went critical in 1943, and when the B Reactor at the Hanford Site did so the next year. At Los Alamos he headed F Division, part of which worked on Edward Teller's thermonuclear "Super" bomb. He was present at the Trinity test on 16 July 1945, where he used his Fermi method to estimate the bomb's yield.
After the war, Fermi served under J. Robert Oppenheimer on the influential General Advisory Committee, which advised the Atomic Energy Commission on nuclear matters and policy. Following the detonation of the first Soviet fission bomb in August 1949, he strongly opposed the development of a hydrogen bomb on both moral and technical grounds. He was among the scientists who testified on Oppenheimer's behalf at the 1954 hearing that resulted in the denial of the latter's security clearance. Fermi did important work in particle physics, especially related to pions and muons, and he speculated that cosmic rays arose through material being accelerated by magnetic fields in interstellar space. Many awards, concepts, and institutions are named after Fermi, including the Enrico Fermi Award, the Enrico Fermi Institute, the Fermi National Accelerator Laboratory, the Fermi Gamma-ray Space Telescope, the Enrico Fermi Nuclear Generating Station, and the synthetic element fermium.
Contents
Early life
Enrico Fermi was born in Rome on 29 September 1901. He was the third child of Alberto Fermi, a division head (Capo Divisione) in the Ministry of Railways, and Ida de Gattis, an elementary school teacher.[5][6] His only sister, Maria, was two years older than he, and his brother, Giulio, was a year older. After the two boys were sent to a rural community to be wet nursed, Enrico rejoined his family in Rome when he was two and a half.[7] While he came from a Roman Catholic family and was baptized in accord with his grandparents' wishes, the family was not religious, and Fermi was an agnostic throughout his adult life. As a young boy he shared his interests with his brother Giulio. They built electric motors and played with electrical and mechanical toys.[8] Giulio died during the administration of anesthesia for an operation on a throat abscess in 1915.[9]One of Fermi's first sources for the study of physics was a book found at the local market of Campo de' Fiori in Rome. The 900-page book from 1840, Elementorum physicae mathematicae, was written in Latin by Jesuit Father Andrea Caraffa, a professor at the Collegio Romano. It covered mathematics, classical mechanics, astronomy, optics, and acoustics, to the extent that they were understood when it was written.[10][11] Fermi befriended another scientifically inclined student, Enrico Persico,[12] and the two worked together on scientific projects such as building gyroscopes and measuring the Earth's magnetic field. Fermi's interest in physics was further encouraged by his father's colleague Adolfo Amidei, who gave him several books on physics and mathematics that he read and assimilated quickly.[13]
Scuola Normale Superiore in Pisa
Fermi graduated from high school in July 1918 and, at Amidei's urging, applied to the Scuola Normale Superiore in Pisa. Having lost one son, his parents were reluctant to let him move away from home for four years while attending the Sapienza University of Rome, but in the end they acquiesced. The school provided free lodging for students, but candidates had to take a difficult entrance exam that included an essay. The given theme was "Specific characteristics of Sounds". The 17-year-old Fermi chose to derive and solve the partial differential equation for a vibrating rod, applying Fourier analysis in the solution. The examiner, Professor Giuseppe Pittarelli from the Sapienza University of Rome, interviewed Fermi and concluded that his entry would have been commendable even for a doctoral degree. Fermi achieved first place in the classification of the entrance exam.[14]During his years at the Scuola Normale Superiore, Fermi teamed up with a fellow student named Franco Rasetti with whom he would indulge in light-hearted pranks and who would later become Fermi's close friend and collaborator. In Pisa, Fermi was advised by the director of the physics laboratory, Luigi Puccianti, who acknowledged that there was little that he could teach Fermi, and frequently asked Fermi to teach him something instead. Fermi's knowledge of quantum physics reached such a high level that Puccianti asked him to organize seminars on the topic.[15] During this time Fermi learned tensor calculus, a mathematical technique invented by Gregorio Ricci and Tullio Levi-Civita that was needed to demonstrate the principles of general relativity.[16] Fermi initially chose mathematics as his major, but soon switched to physics. He remained largely self-taught, studying general relativity, quantum mechanics, and atomic physics.[17]

A light cone is a three-dimensional surface of all possible light rays arriving at and departing from a point in spacetime. Here, it is depicted with one spatial dimension suppressed. The time line is the vertical axis.
The first paper seemed to point out a contradiction between the electrodynamic theory and the relativistic one concerning the calculation of the electromagnetic masses, as the former predicted a value of 4/3 U/c2. Fermi addressed this the next year in a paper "Concerning a contradiction between electrodynamic and the relativistic theory of electromagnetic mass" in which he showed that the apparent contradiction was a consequence of relativity. This paper was sufficiently well-regarded that it was translated into German and published in the German scientific journal Physikalische Zeitschrift in 1922.[19] That year, Fermi submitted his article "On the phenomena occurring near a world line" (Italian: Sopra i fenomeni che avvengono in vicinanza di una linea oraria) to the Italian journal I Rendiconti dell'Accademia dei Lincei. In this article he examined the Principle of Equivalence, and introduced the so-called "Fermi coordinates". He proved that on a world line close to the time line, space behaves as if it were a Euclidean space.[20][21]
Fermi submitted his thesis, "A theorem on probability and some of its applications" (Italian: Un teorema di calcolo delle probabilità ed alcune sue applicazioni), to the Scuola Normale Superiore in July 1922, and received his laurea at the unusually young age of 21. The thesis was on X-ray diffraction images. Theoretical physics was not yet considered a discipline in Italy, and the only thesis that would have been accepted was one on experimental physics. For this reason, Italian physicists were slow in embracing the new ideas like relativity coming from Germany. Since Fermi was quite at home in the lab doing experimental work, this did not pose insurmountable problems for him.[21]

Fermi–Dirac statistics. Fermi function F( ) vs. energy
) vs. energy  , with μ = 0.55 eV and for various temperatures in the range 50 to 375 K (−223.2 to 101.9 °C).
, with μ = 0.55 eV and for various temperatures in the range 50 to 375 K (−223.2 to 101.9 °C).
 ) vs. energy
) vs. energy  , with μ = 0.55 eV and for various temperatures in the range 50 to 375 K (−223.2 to 101.9 °C).
, with μ = 0.55 eV and for various temperatures in the range 50 to 375 K (−223.2 to 101.9 °C).Fermi decided to travel abroad, and spent a semester studying under Max Born at the University of Göttingen, where he met Werner Heisenberg and Pascual Jordan. Fermi then studied in Leiden with Paul Ehrenfest from September to December 1924 on a fellowship from the Rockefeller Foundation obtained through the intercession of the mathematician Vito Volterra. Here Fermi met Hendrik Lorentz and Albert Einstein, and became good friends with Samuel Goudsmit and Jan Tinbergen. From January 1925 to late 1926, Fermi taught mathematical physics and theoretical mechanics at the University of Florence, where he teamed up with Rasetti to conduct a series of experiments on the effects of magnetic fields on mercury vapour. He also participated in seminars at the Sapienza University of Rome, giving lectures on quantum mechanics and solid state physics.[22] While giving lectures of new quantum mechanics based on remarkable accuracy of predictions of Schrödinger equation, the Italian physicist would often say, "It has no business to fit so well!"[23]
After Wolfgang Pauli announced his exclusion principle in 1925, Fermi responded with a paper "On the quantisation of the perfect monoatomic gas" (Italian: Sulla quantizzazione del gas perfetto monoatomico), in which he applied the exclusion principle to an ideal gas. The paper was especially notable for Fermi's statistical formulation, which describes the distribution of particles in systems of many identical particles that obey the exclusion principle. This was independently developed soon after by the British physicist Paul Dirac, who also showed how it was related to the Bose–Einstein statistics. Accordingly, it is now known as Fermi–Dirac statistics.[24] Following Dirac, particles that obey the exclusion principle are today called "fermions", while those that do not are called "bosons".[25]
Professor in Rome
Professorships in Italy were granted by competition (Italian: concorso) for a vacant chair, the applicants being rated on their publications by a committee of professors. Fermi applied for a chair of mathematical physics at the University of Cagliari on Sardinia, but was narrowly passed over in favour of Giovanni Giorgi.[26] In 1926, at the age of 24, he applied for a professorship at the Sapienza University of Rome. This was a new chair, one of the first three in theoretical physics in Italy, that had been created by the Minister of Education at the urging of Professor Orso Mario Corbino, who was the University's professor of experimental physics, the Director of the Institute of Physics, and a member of Benito Mussolini's cabinet. Corbino, who also chaired the selection committee, hoped that the new chair would raise the standard and reputation of physics in Italy.[27] The committee chose Fermi ahead of Enrico Persico and Aldo Pontremoli,[28] and Corbino helped Fermi recruit his team, which was soon joined by notable students such as Edoardo Amaldi, Bruno Pontecorvo, Ettore Majorana and Emilio Segrè, and by Franco Rasetti, whom Fermi had appointed as his assistant.[29] They were soon nicknamed the "Via Panisperna boys" after the street where the Institute of Physics was located.[30]
Fermi and his students (the Via Panisperna boys) in the courtyard of Rome University's Physics Institute in Via Panisperna, about 1934. From Left to right: Oscar D'Agostino, Emilio Segrè, Edoardo Amaldi, Franco Rasetti and Fermi
During their time in Rome, Fermi and his group made important contributions to many practical and theoretical aspects of physics. In 1928, he published his Introduction to Atomic Physics (Italian: Introduzione alla fisica atomica), which provided Italian university students with an up-to-date and accessible text. Fermi also conducted public lectures and wrote popular articles for scientists and teachers in order to spread knowledge of the new physics as widely as possible.[37] Part of his teaching method was to gather his colleagues and graduate students together at the end of the day and go over a problem, often from his own research.[37][38] A sign of success was that foreign students now began to come to Italy. The most notable of these was the German physicist Hans Bethe,[39] who came to Rome as a Rockefeller Foundation fellow, and collaborated with Fermi on a 1932 paper "On the Interaction between Two Electrons" (German: Über die Wechselwirkung von Zwei Elektronen).[37]
At this time, physicists were puzzled by beta decay, in which an electron was emitted from the atomic nucleus. To satisfy the law of conservation of energy, Pauli postulated the existence of an invisible particle with no charge and little or no mass that was also emitted at the same time. Fermi took up this idea, which he developed in a tentative paper in 1933, and then a longer paper the next year that incorporated the postulated particle, which Fermi called a "neutrino".[40][41][42] His theory, later referred to as Fermi's interaction, and still later as the theory of the weak interaction, described one of the four fundamental forces of nature. The neutrino was detected after his death, and his interaction theory showed why it was so difficult to detect. When he submitted his paper to the British journal Nature, that journal's editor turned it down because it contained speculations which were "too remote from physical reality to be of interest to readers".[41] Thus Fermi saw the theory published in Italian and German before it was published in English.[29]
In the introduction to the 1968 English translation, physicist Fred L. Wilson noted that:
Fermi's theory, aside from bolstering Pauli's proposal of the neutrino, has a special significance in the history of modern physics. One must remember that only the naturally occurring β emitters were known at the time the theory was proposed. Later when positron decay was discovered, the process was easily incorporated within Fermi's original framework. On the basis of his theory, the capture of an orbital electron by a nucleus was predicted and eventually observed. With time much experimental data has accumulated. Although peculiarities have been observed many times in β decay, Fermi's theory always has been equal to the challenge.In January 1934, Irène Joliot-Curie and Frédéric Joliot announced that they had bombarded elements with alpha particles and induced radioactivity in them.[43][44] By March, Fermi's assistant Gian-Carlo Wick had provided a theoretical explanation using Fermi's theory of beta decay. Fermi decided to switch to experimental physics, using the neutron, which James Chadwick had discovered in 1932.[45] In March 1934, Fermi wanted to see if he could induce radioactivity with Rasetti's polonium-beryllium neutron source. Neutrons had no electric charge, and so would not be deflected by the positively charged nucleus. This meant that they needed much less energy to penetrate the nucleus than charged particles, and so would not require a particle accelerator, which the Via Panisperna boys did not have.[46][47]
The consequences of the Fermi theory are vast. For example, β spectroscopy was established as a powerful tool for the study of nuclear structure. But perhaps the most influential aspect of this work of Fermi is that his particular form of the β interaction established a pattern which has been appropriate for the study of other types of interactions. It was the first successful theory of the creation and annihilation of material particles. Previously, only photons had been known to be created and destroyed.[42]
Fermi had the idea to resort to replacing the polonium-beryllium neutron source with a radon-beryllium one, which he created by filling a glass bulb with beryllium powder, evacuating the air, and then adding 50 mCi of radon gas, supplied by Giulio Cesare Trabacchi.[48][49] This created a much stronger neutron source, the effectiveness of which declined with the 3.8-day half-life of radon. He knew that this source would also emit gamma rays, but, on the basis of his theory, he believed that this would not affect the results of the experiment. He started by bombarding platinum, an element with a high atomic number that was readily available, without success. He turned to aluminium, which emitted an alpha particle and produced sodium, which then decayed into magnesium by beta particle emission. He tried lead, without success, and then fluorine in the form of calcium fluoride, which emitted an alpha particle and produced nitrogen, decaying into oxygen by beta particle emission. In all, he induced radioactivity in 22 different elements.[50] Fermi rapidly reported the discovery of neutron-induced radioactivity in the Italian journal La Ricerca Scientifica on 25 March 1934.[49][51][52]

Beta decay. A neutron decays into a proton, and an electron is emitted. In order for the total energy in the system to remain the same, Pauli and Fermi postulated that a neutrino ( ) was also emitted
) was also emitted
 ) was also emitted
) was also emittedThe Via Panisperna boys also noticed some unexplained effects. The experiment seemed to work better on a wooden table than a marble table top. Fermi remembered that Joliot-Curie and Chadwick had noted that paraffin wax was effective at slowing neutrons, so he decided to try that. When neutrons were passed through paraffin wax, they induced a hundred times as much radioactivity in silver compared with when it was bombarded without the paraffin. Fermi guessed that this was due to the hydrogen atoms in the paraffin. Those in wood similarly explained the difference between the wooden and the marble table tops. This was confirmed by repeating the effect with water. He concluded that collisions with hydrogen atoms slowed the neutrons.[55][47] The lower the atomic number of the nucleus it collides with, the more energy a neutron loses per collision, and therefore the less collisions that are required to slow a neutron down by a given amount.[56] Fermi realised that this induced more radioactivity because slow neutrons were more easily captured than fast ones. He developed a diffusion equation to describe this, which became known as the Fermi age equation.[55][47]
In 1938 Fermi received the Nobel Prize in Physics at the age of 37 for his "demonstrations of the existence of new radioactive elements produced by neutron irradiation, and for his related discovery of nuclear reactions brought about by slow neutrons".[57] After Fermi received the prize in Stockholm, he did not return home to Italy, but rather continued on to New York City along with his family, where they applied for permanent residency. The decision to move to America and become US citizens was primarily a result of the racial laws in Italy.[33]
Manhattan Project
Soon after Fermi's arrival in New York City on 2 January 1939,[58] he was offered five different chairs, and chose to work at Columbia University,[59] where he had already given summer lectures in 1936.[60] He received the news that in December 1938, the German chemists Otto Hahn and Fritz Strassmann had detected the element barium after bombarding uranium with neutrons,[61] which Lise Meitner and her nephew Otto Frisch correctly interpreted as the result of nuclear fission. Frisch confirmed this experimentally on 13 January 1939.[62][63] The news of Meitner and Frisch's interpretation of Hahn and Strassmann's discovery crossed the Atlantic with Niels Bohr, who was to lecture at Princeton University. Isidor Isaac Rabi and Willis Lamb, two Columbia University physicists working at Princeton, found out about it and carried it back to Columbia. Rabi said he told Enrico Fermi, but Fermi later gave the credit to Lamb:[64]I remember very vividly the first month, January, 1939, that I started working at the Pupin Laboratories because things began happening very fast. In that period, Niels Bohr was on a lecture engagement at the Princeton University and I remember one afternoon Willis Lamb came back very excited and said that Bohr had leaked out great news. The great news that had leaked out was the discovery of fission and at least the outline of its interpretation. Then, somewhat later that same month, there was a meeting in Washington where the possible importance of the newly discovered phenomenon of fission was first discussed in semi-jocular earnest as a possible source of nuclear power.[65]Noddack was proven right after all. Fermi had dismissed the possibility of fission on the basis of his calculations, but he had not taken into account the binding energy that would appear when a nuclide with an odd number of neutrons absorbed an extra neutron.[54] For Fermi, the news came as a profound embarrassment, as the transuranic elements that he had partly been awarded the Nobel Prize for discovering had not been transuranic elements at all, but fission products. He added a footnote to this effect to his Nobel Prize acceptance speech.[66][64]
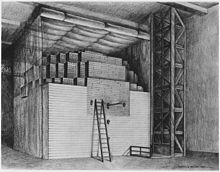
Illustration of Chicago Pile-1,
the first nuclear reactor to achieve a self-sustaining chain reaction.
Designed by Fermi. it consisted of uranium and uranium oxide in a cubic
lattice embedded in graphite.
Few weeks after French scientists Hans von Halban, Lew Kowarski and Frédéric Joliot-Curie,[69] Fermi and Anderson demonstrated that uranium bombarded by neutrons emitted more neutrons than it absorbed.[70][71] Leó Szilárd obtained 200 kilograms (440 lb) of uranium oxide from Canadian radium producer Eldorado Gold Mines Limited, allowing Fermi and Anderson to conduct experiments with fission on a much larger scale.[72] Fermi and Szilárd collaborated on a design of a device to achieve a self-sustaining nuclear reaction—a nuclear reactor. Due to the rate of absorption of neutrons by the hydrogen in water, it was unlikely that a self-sustaining reaction could be achieved with natural uranium and water as a neutron moderator. Fermi suggested, based on his work with neutrons, that uranium oxide could be used in the form of blocks, with graphite as a moderator instead of water. This would reduce the rate of capture of the neutrons, and make it theoretically possible to achieve a self-sustaining chain reaction. Szilárd then came up with what proved to be a workable design, a pile of uranium oxide blocks surrounded by graphite bricks.[73] Szilárd, Anderson and Fermi jointly published a paper on "Neutron Production in Uranium" [72] but their work habits and personalities were different, and Fermi had trouble working with Szilárd.[74]
Fermi was the first to warn military leaders about the potential impact of nuclear energy, giving a lecture on the subject at the Navy Department on 18 March 1939. The response fell short of what he had hoped for, although the Navy agreed to provide $1,500 towards further research at Columbia.[75] In August 1939, three Hungarian physicists—Szilárd, Eugene Wigner and Edward Teller—prepared the Einstein–Szilárd letter, which they persuaded Einstein to sign, warning President Franklin D. Roosevelt of the probability that Germany was planning to build an atomic bomb. Because of the German invasion of Poland on 1 September, it was October before they could arrange for the letter to be personally delivered. Roosevelt was sufficiently concerned that he assembled the S-1 Uranium Committee to investigate the matter.[76]

Fermi's ID badge photo from Los Alamos
Given the number of unknown factors involved in creating a self-sustaining nuclear reaction, it seemed inadvisable to do so in a densely populated area. Compton arranged with Colonel Kenneth Nichols, the head of the Army's Manhattan District, for land to be acquired in the Argonne Forest about 20 miles (32 km) from Chicago, and Stone & Webster was contracted to develop the site. This work was halted by an industrial dispute. Fermi then persuaded Compton that he could build a reactor in the squash court under Stagg Field at the University of Chicago. Construction of the pile began on 6 November 1942, and Chicago Pile-1 went critical on 2 December.[82] The shape of the pile was intended to be roughly spherical, but as work proceeded Fermi calculated that criticality could be achieved without finishing the entire pile as planned.[83]
This experiment was a landmark in the quest for energy, and it was typical of Fermi's approach. Every step was carefully planned, every calculation meticulously done.[82] When the first self-sustained nuclear chain reaction was achieved, Compton made a coded phone call to James B. Conant, the chairman of the National Defense Research Committee.
I picked up the phone and called Conant. He was reached at the President's office at Harvard University. "Jim," I said, "you'll be interested to know that the Italian navigator has just landed in the new world." Then, half apologetically, because I had led the S-l Committee to believe that it would be another week or more before the pile could be completed, I added, "the earth was not as large as he had estimated, and he arrived at the new world sooner than he had expected."
"Is that so," was Conant's excited response. "Were the natives friendly?" "Everyone landed safe and happy."[84]

Fermi (centre), with Ernest O. Lawrence (left) and Isidor Isaac Rabi (right)
Just in case something went wrong, Fermi was on hand at Oak Ridge to witness the air-cooled X-10 Graphite Reactor go critical on 4 November 1943. The technicians woke him early so that he could see it happen.[87] Getting X-10 operational was another milestone in the plutonium project. It provided data on reactor design, training for DuPont staff in reactor operation, and produced the first small quantities of reactor-bred plutonium.[88] Fermi became an American citizen in July 1944, the earliest date the law allowed.[89]
In September 1944, Fermi inserted the first uranium fuel slug into the B Reactor at the Hanford Site, the production reactor designed to breed plutonium in large quantities. Like X-10, it had been designed by Fermi's team at the Metallurgical Laboratory, and built by DuPont, but it was much larger, and was water-cooled. Over the next few days, 838 tubes were loaded, and the reactor went critical. Shortly after midnight on 27 September, the operators began to withdraw the control rods to initiate production. At first all appeared to be well, but around 03:00, the power level started to drop and by 06:30 the reactor had shut down completely. The Army and DuPont turned to Fermi's team for answers. The cooling water was investigated to see if there was a leak or contamination. The next day the reactor suddenly started up again, only to shut down once more a few hours later. The problem was traced to neutron poisoning from xenon-135, a fission product with a half-life of 9.2 hours. Fortunately, DuPont had deviated from the Metallurgical Laboratory's original design in which the reactor had 1,500 tubes arranged in a circle, and had added an additional 504 tubes to fill in the corners. The scientists had originally considered this over-engineering a waste of time and money, but Fermi realized that by loading all 2,004 tubes, the reactor could reach the required power level and efficiently produce plutonium.[90][91]

The FERMIAC, an analog device invented by Enrico Fermi to implement studies of neutron transport
Along with Oppenheimer, Compton and Ernest Lawrence, Fermi was part of the scientific panel that advised the Interim Committee on target selection. The panel agreed with the committee that atomic bombs be used without warning against an industrial target.[95] Like others at the Los Alamos Laboratory, Fermi found out about the atomic bombings of Hiroshima and Nagasaki from the public address system in the technical area. Fermi did not believe that atomic bombs would scare people into not starting wars, nor did he think that the time was ripe for world government. He therefore did not join the Association of Los Alamos Scientists.[96]
Post-war work
Fermi became a professor at the University of Chicago on 1 July 1945,[97] although he did not depart the Los Alamos Laboratory with his family until 31 December 1945.[98] The Metallurgical Laboratory became the Argonne National Laboratory on 1 July 1946, the first of the national laboratories established by the Manhattan Project.[99] The short distance between Chicago and Argonne allowed Fermi to work at both places. At Argonne he continued experimental physics, investigating neutron scattering with Leona Marshall.[100] He also discussed theoretical physics with Maria Mayer, helping her develop insights into spin–orbit coupling that would lead to her receiving the Nobel Prize.[101]The Manhattan Project was replaced by Atomic Energy Commission (AEC) on 1 January 1947.[102] Fermi served on the AEC General Advisory Committee, an influential scientific committee chaired by Robert Oppenheimer.[103] He also liked to spend a few weeks of each year at the Los Alamos National Laboratory,[104] where he collaborated with Nicholas Metropolis,[105] and with John von Neumann on Rayleigh–Taylor instability, the science of what occurs at the border between two fluids of different densities.[106]
Following the detonation of the first Soviet fission bomb in August 1949, Fermi, along with Isidor Rabi, wrote a strongly worded report for the committee, opposing the development of a hydrogen bomb on moral and technical grounds.[107] Nonetheless, Fermi continued to participate in work on the hydrogen bomb at Los Alamos as a consultant. Along with Stanislaw Ulam, he calculated that not only would the amount of tritium needed for Teller's model of a thermonuclear weapon be prohibitive, but a fusion reaction could still not be assured to propagate even with this large quantity of tritium.[108] Fermi was among the scientists who testified on Oppenheimer's behalf at the Oppenheimer security hearing in 1954 that resulted in denial of Oppenheimer's security clearance.[109]
In his later years, Fermi continued teaching at the University of Chicago. His PhD students in the post-war period included Owen Chamberlain, Geoffrey Chew, Jerome Friedman, Marvin Goldberger, Tsung-Dao Lee, Arthur Rosenfeld and Sam Treiman.[1][110] Jack Steinberger was a graduate student.[111] Fermi conducted important research in particle physics, especially related to pions and muons. He made the first predictions of pion-nucleon resonance,[105] relying on statistical methods, since he reasoned that exact answers were not required when the theory was wrong anyway.[112] In a paper co-authored with Chen Ning Yang, he speculated that pions might actually be composite particles.[113] The idea was elaborated by Shoichi Sakata. It has since been supplanted by the quark model, in which the pion is made up of quarks, which completed Fermi's model, and vindicated his approach.[114]
Fermi wrote a paper "On the Origin of Cosmic Radiation" in which he proposed that cosmic rays arose through material being accelerated by magnetic fields in interstellar space, which led to a difference of opinion with Teller.[112] Fermi examined the issues surrounding magnetic fields in the arms of a spiral galaxy.[115] He mused about what is now referred to as the "Fermi paradox": the contradiction between the presumed probability of the existence of extraterrestrial life and the fact that contact has not been made.[116]
Toward the end of his life, Fermi questioned his faith in society at large to make wise choices about nuclear technology. He said:
Some of you may ask, what is the good of working so hard merely to collect a few facts which will bring no pleasure except to a few long-haired professors who love to collect such things and will be of no use to anybody because only few specialists at best will be able to understand them? In answer to such question[s] I may venture a fairly safe prediction. History of science and technology has consistently taught us that scientific advances in basic understanding have sooner or later led to technical and industrial applications that have revolutionized our way of life. It seems to me improbable that this effort to get at the structure of matter should be an exception to this rule. What is less certain, and what we all fervently hope, is that man will soon grow sufficiently adult to make good use of the powers that he acquires over nature.[117]Fermi died at age 53 of stomach cancer in his home in Chicago,[4] and was interred at Oak Woods Cemetery.[118]
A commemorative plaque remembers him in the Basilica of Santa Croce, Florence, a church also known as "Temple of Italian Glories", for the many burials of artists, scientists and prominent figures in Italian history.[119]
Impact and legacy
As a person, Fermi seemed simplicity itself. He was extraordinarily
vigorous and loved games and sport. On such occasions his ambitious
nature became apparent. He played tennis with considerable ferocity and
when climbing mountains acted rather as a guide. One might have called
him a benevolent dictator. I remember once at the top of a mountain
Fermi got up and said: "Well, it is two minutes to two, let's all leave
at two o'clock"; and of course, everybody got up faithfully and
obediently. This leadership and self-assurance gave Fermi the name of
"The Pope" whose pronouncements were infallible in physics. He once
said: "I can calculate anything in physics within a factor 2 on a few
sheets: to get the numerical factor in front of the formula right may
well take a physicist a year to calculate, but I am not interested in
that." His leadership could go so far that it was a danger to the
independence of the person working with him. I recollect once, at a
party at his house when my wife cut the bread, Fermi came along and said
he had a different philosophy on bread-cutting and took the knife out
of my wife's hand and proceeded with the job because he was convinced
that his own method was superior. But all this did not offend at all,
but rather charmed everybody into liking Fermi. He had very few
interests outside physics and when he once heard me play on Teller's
piano he confessed that his interest in music was restricted to simple
tunes.
Egon Bretscher[3]
Legacy
Fermi received numerous awards in recognition of his achievements, including the Matteucci Medal in 1926, the Nobel Prize for Physics in 1938, the Hughes Medal in 1942, the Franklin Medal in 1947, and the Rumford Prize in 1953. He was awarded the Medal for Merit in 1946 for his contribution to the Manhattan Project.[120] In 1999, Time named Fermi on its list of the top 100 persons of the twentieth century.[121] Fermi was widely regarded as an unusual case of a 20th-century physicist who excelled both theoretically and experimentally. The historian of physics, C. P. Snow, wrote that "if Fermi had been born a few years earlier, one could well imagine him discovering Rutherford's atomic nucleus, and then developing Bohr's theory of the hydrogen atom. If this sounds like hyperbole, anything about Fermi is likely to sound like hyperbole".[122]Fermi was known as an inspiring teacher, and was noted for his attention to detail, simplicity, and careful preparation of his lectures.[123] Later, his lecture notes were transcribed into books.[124] His papers and notebooks are today in the University of Chicago.[125] Victor Weisskopf noted how Fermi "always managed to find the simplest and most direct approach, with the minimum of complication and sophistication."[126] Fermi's ability and success stemmed as much from his appraisal of the art of the possible, as from his innate skill and intelligence. He disliked complicated theories, and while he had great mathematical ability, he would never use it when the job could be done much more simply. He was famous for getting quick and accurate answers to problems that would stump other people. Later on, his method of getting approximate and quick answers through back-of-the-envelope calculations became informally known as the "Fermi method", and is widely taught.[127]
Fermi was fond of pointing out that Alessandro Volta, working in his laboratory, could have had no idea where the study of electricity would lead.[128] Fermi is generally remembered for his work on nuclear power and nuclear weapons, especially the creation of the first nuclear reactor, and the development of the first atomic and hydrogen bombs. His scientific work has stood the test of time. This includes his theory of beta decay, his work with non-linear systems, his discovery of the effects of slow neutrons, his study of pion-nucleon collisions, and his Fermi–Dirac statistics. His speculation that a pion was not a fundamental particle pointed the way towards the study of quarks and leptons.[129]
Things named after Fermi
Main article: List of things named after Enrico Fermi
Many things have been named in Fermi's honour. These include the Fermilab particle accelerator and physics lab in Batavia, Illinois, which was renamed in his honour in 1974,[130] and the Fermi Gamma-ray Space Telescope, which was named after him in 2008, in recognition of his work on cosmic rays.[131] Three nuclear reactor installations have been named after him: the Fermi 1 and Fermi 2 nuclear power plants in Newport, Michigan, the Enrico Fermi Nuclear Power Plant at Trino Vercellese in Italy,[132] and the RA-1 Enrico Fermi research reactor in Argentina.[133] A synthetic element isolated from the debris of the 1952 Ivy Mike nuclear test was named fermium, in honour of Fermi's contributions to the scientific community. It follows the element einsteinium, which was discovered with it.[134][135] Since 1956, the United States Atomic Energy Commission has named its highest honour, the Fermi Award,
after him. Recipients of the award include well-known scientists like
Otto Hahn, Robert Oppenheimer, Edward Teller and Hans Bethe.[136]Bibliography
- Introduzione alla Fisica Atomica (in Italian). Bologna: N. Zanichelli. 1928. OCLC 9653646.
- Fisica per i Licei (in Italian). Bologna: N. Zanichelli. 1929. OCLC 9653646.
- Molecole e cristalli (in Italian). Bologna: N. Zanichelli. 1934. OCLC 19918218.
- Thermodynamics. New York: Prentice Hall. 1937. OCLC 2379038.
- Fisica per Istituti Tecnici (in Italian). Bologna: N. Zanichelli. 1938.
- Fisica per Licei Scientifici (in Italian). Bologna: N. Zanichelli. 1938.
- Elementary particles. New Haven: Yale University Press. 1951. OCLC 362513.
Patents
- US Patent 2206634, "Process for the Production of Radioactive Substances", issued July 1940
- US Patent 2836554, "Air Cooled Neutronic Reactor", issued April 1950
- US Patent 2524379, "Neutron Velocity Selector", issued October 1950
- US Patent 2852461, "Neutronic Reactor", issued September 1953
- US Patent 2708656, "Neutronic Reactor", issued May 1955
- US Patent 2768134, "Testing Material in a Neutronic Reactor", issued October 1956
- US Patent 2780595, "Test Exponential Pile", issued February 1957
- US Patent 2798847, "Method of Operating a Neutronic Reactor", issued July 1957
- US Patent 2807581, "Neutronic Reactor", issued September 1957
- US Patent 2807727, "Neutronic Reactor Shield", issued September 1957
- US Patent 2813070, "Method of Sustaining a Neutronic Chain Reacting System", issued November 1957
- US Patent 2837477, "Chain Reacting System", issued June 1958
- US Patent 2931762, "Neutronic Reactor", issued April 1960
- US Patent 2969307, "Method of Testing Thermal Neutron Fissionable Material for Purity", issued January 1961



No comments:
Post a Comment
Please leave a comment-- or suggestions, particularly of topics and places you'd like to see covered